Available Factors in Korea
Global leader contributing
to Hemophilia Patients’ lives
Development of Factor Concentrates
| Before 1974 | Whole plasma or FFP were used for hemophilia treatment |
|---|---|
| Some patients could use personal imported factor concentrates | |
| 1974 | AHF was produced by Korea Green Cross Co. |
| 1987~1995 | Octa-Ⅵ (ion change high purify Factor Ⅷ) was produced by Korea Green Cross Co. |
| Octa-Ⅵ using TNBP was produced 1989. It changes the name 'Green Eight' 1995 | |
| Facnyne (pdFⅨ) was produced by Korea Green Cross Co. | |
| 1998 | FEIBA (aPCC) was imported |
| 2000 | GreenMono (pdFⅧ, monoclonal Ab, S/D, +albumin) was produced by Green Cross Co. |
| Monoclate-P (pdFⅧ), NovoSeven (rⅦa) were imported | |
| 2003 | Recombinant factor concentrates were imported (Recombinate, BeneFix) |
| 2005 | Immunate (pdFⅧ+vWF) was imported |
| 2009 | ADVATE (rFⅧ) was imported |
| 2010 | Kogenate FS (rFⅧ) was imported |
| 2011 | GreenGene F (rFⅧ, no albumin) was produced by Green Cross Co. |
| 2012 | Xyntha (rFⅧ) was imported |
| 2016 | Rixubis (rFⅨ) was imported |
| 2018 | Adynovate(rFⅧ) was imported |
Domestic factor concentrates
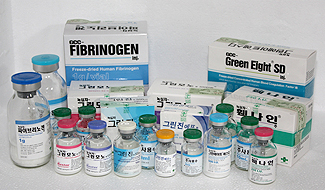 |
GreenGene F : rFⅧ, no albumin |
|---|---|
| GreenMono : FⅧ, monoclonal Ab, S/D, + albumin | |
| GreenEight : FⅧ & vWF, chromatography, S/D, no albumin | |
| Facnyne : PCC, S/D | |
| Greenplast : fibrin glue |
Imported factor concentrates
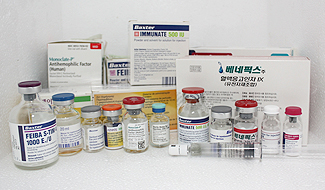 |
Monoclate-P : FⅧ monoclonal Ab, pasteurization, +albumin |
|---|---|
| Advate, Koginate FS, Xyntha: rFⅧ | |
| BeneFix , Rixubis : rFⅨ | |
| FEIBA : aPCC | |
| NovoSeven : rFⅦa | |
| Immunate : pd FⅧ+vWF |